Electrochemical corrosion
Each metal is characterised by its own electrical potential. Thus, electrical contact between two metals with different potentials induces a transfer of current resulting in an electrochemical reaction. Over time, this reaction can lead to corrosion at the contact between the two metals.
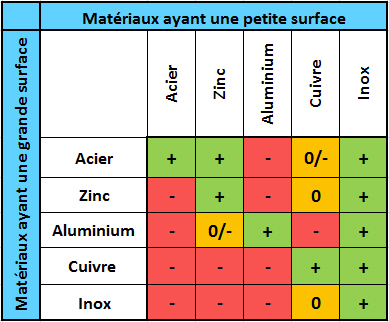
Electronic compatibility of metals in ambient air inducing electrochemical corrosion + : Good | 0 : Uncertain | - : Poor
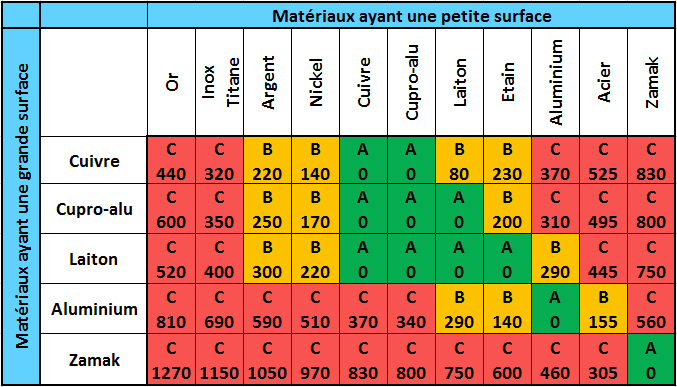
Electrochemical compatibility of metals in a 2% salt bath inducing electrochemical corrosion A: Good | B: Fair | C: Poor

